Europe not hungry for GM potatoes - EU Agriculture Council rejects latest attempt to grow GMOs in Europe
CRIIGEN answers to European Food Safety Authority critique of MON 863 study
EFSA fails to protect European citizens from a risky GM maize
Irish Government and Doctors Position
Greece Extends Biotech Ban
Monsanto maize approved for human consumption potentially toxic, warns new study
EU stands up to US pressure
Cyprus to Shelve GM Food Separately
IRELAND AIMS TO BECOME A GMO-FREE ZONE
Measures and actions to protect Cyprus from GMOs
More EU states wary on GMO maize
GM and non-GM crops too close, study says
US still bullying EU to market GMOs
France could follow Germany in GM corn restriction
MEPs CALL ON THE COMMISSION TO BAN THE INTRODUCTION OF GMOs
Germany Tightens Restrictions on Genetically Modified Corn
Netherlands refuses GM corn shipment from US
ILLEGAL GM MAIZE ENTERS EU THROUGH IRELAND
German group denied permit to grow GM rapeseed in Lithuania
LAW ON FOOD (Croatia): HSP Against Importing GMO Products
Russia joins the battle over GM products
EFSA to review Monsanto maize concerns
More Doubts About Genetically Modified Corn
New setback for biotech crops in Europe
GM SPUDS HAVE HAD THEIR CHIPS AS IRISH TRIALS STOPPED
Dutch Council of State ordered destruction of BASF GE potato field trial
GM starch potato: still no cultivation in 2007
GM CROPS FAIL EU LISBON AGENDA GOALS
European ministers uphold Hungary's right to ban GMO crop
EU ENVIRONMENT MINISTERS TWICE RESIST GMOs
ONE MILLION EU CITIZENS CALL FOR LABELLING OF GM FOODS
Europe resists takeover by biotech crops
Europe not hungry for GM potatoes - EU Agriculture Council rejects latest attempt to grow GMOs in Europe
Friends of the Earth Europe, 16th July 2007
Friends of the Earth Europe has welcomed EU member states' rejection of the latest application to grow GMOs in Europe, as the EU Agriculture Council today failed to approve the commercial growing of a genetically modified potato. There have now been no new GMOs grown in the EU for ten years. Helen Holder, GMO Campaigner at Friends of the Earth Europe said: "Too few EU member states support growing genetically modified crops, and now yet another has been refused authorisation. National governments recognised the safety risks of growing this GM potato, as they have with previous applications. Now the decision is in the hands of the European Commission and we urge it to reject it too."
Today's vote was on an application to grow the genetically modified potato for use in industrial processes like making paper. The producer - German chemicals giant BASF - has also applied for approval to use the same potato in food and animal feed and acknowledges that contamination of the food chain is possible. The European Food Safety Authority (EFSA) gave the GM potato the green light, but has been criticized for overlooking several important health and environmental risks:
* Antibiotic resistance marker gene: the potato contains a gene which can convey resistance to antibiotics. Under EU law, genes of this kind should have been phased out by the end of 2004. EFSA acknowledges that the cultivation of this potato could lead to antibiotic resistance, yet argued that this did not pose a "relevant" risk to human health or to the environment.
* The risk assessment, required under EU law, fails to fulfil legal requirements. Basic information on the health and environmental safety of the GM potato is missing; in particular there is only an analysis of effects of surrounding wildlife on the potato, rather than looking at the impact of the GM potato on the environment.
* Effects on health have not been sufficiently investigated. A number of irregularities, including toxicological differences that could have serious implications for food safety, have simply not been probed either by BASF or by EFSA
* BASF admits that food contamination is likely: the potato has been genetically modified by the chemical giant BASF to increase its amylopectin content, which is used to produce starch. Although it is not intended to enter the food chain, BASF have issued a separate application for use in human food and animal feed, stating that "it cannot be excluded that amylopectin potato.. may be used as or may be present in food" [2].
* The risk of contaminating future crops is ignored. As they grow underground, it is virtually impossible to harvest all potatoes from a crop. Potatoes therefore grow back the following years and future crops could be contaminated with the genetically modified variant.
"No new GMOs have been grown in the European Union for 10 years now and research show that GMOs actually stimulate the economy less than green farming measures. It is time to accept that there is simply no market for genetically modified crops."
"The big GMO companies claim that using genetically modified potatoes in industrial processes is an environmentally-friendly option, but this is absurd considering the associated health and environmental risks," Ms Holder added.
For more information, please contact:
Rosemary Hall, Communications Officer at Friends of the Earth Europe: Mobile +32 485 930515, rosemary.hall@foeeurope.org
Helen Holder, Coordinator of the Friends of the Earth Europe GMOs campaign: Mobile +32 474 857638, helen.holder@foeeurope.org
Notes:
[1] Application for cultivation of Amylopectin Potato Event EH92-527-1 according to Directive 2001/18
[2] Application for Amylopectin Potato Event EH92-527-1 according to Regulation (EC) No 1829/2003, BASF Plant Sciences.
CRIIGEN answers to European Food Safety Authority critique of MON 863 study - July 2007
Comite de Recherche et d'Information Independantes sur le Genie Genetique Siege Social : 40, rue de Monceau. 75008 Paris
criigen@unicaen.fr - www.criigen.org
In June 2007, the European Food Safety Authority (EFSA) issued a press release[1] describing recent analyses of our publication[2] in an international journal. Our study represents to date the most detailed statistical peer-reviewed paper on one of the longest toxicological studies on a commercialized GMO. After careful consideration of the June 28 EFSA review on the GM maize MON 863 toxicological test, we indicate here our 5 main points of disagreement for the international scientific community, government authorities and the public. These are listed below by order of importance with EFSA views and supporting organisations or societies:
1. We disapprove the politics of absence of transparency on the original crude data of the toxicological tests accepted by EFSA. Normally, all scientists should have access to all blood, urine, organs and weight analyses for rats eating a commercialized GMO. Accepting such data as confidential, EFSA blocks the normal peer-review functioning of science and the knowledge of any toxicological effect not seen before. We recall that this was the case for MON 863, and there was a Court Appeal by Monsanto Company in Germany to avoid the public data release. The case was lost. The Directive CEE/2001/18 asks for transparency of environmental and health impacts of GMOs. Having in its confidential files similar data for other GMOs, EFSA blocks the scientific comparisons that could allow a better understanding of what happens after MON 863 consumption on mammalian physiology. This is very important.
2. CRIIGEN disagrees with EFSA in accepting the fact that GMOs are safe for humans and farm mammals, if detailed blood and numerous organ analyses are available only for rats at a mammalian level. This is also a crucial point and a scientific weakness that EFSA wrongly accepts. It is serious, even if scarce nutritional data are there in addition on other species. We note that for pesticides and drugs, at least three mammalian species are studied at a detailed toxicological level, before commercialization. This MON 863 GMO and more than 99% cultivated commercialized GMOs do contain new pesticide residues that have unknown effects on human an animal health.
3. CRIIGEN disagrees with the fact that EFSA accepts 90-day feeding trials for mammals as the longest necessary from a toxicological point of view (with detailed analyses on numerous organs). The current scientific void regarding safety of GMOs without longer feeding trials (two-year tests, tests run for the duration of a lifespan, and tests on more than one generation). EFSA supports this lack of knowledge, even though it has the capacity to ask for more data from companies before commercialization. As a result, EFSA has accepted an enormous responsibility in case of any accident or chronic effect of a GMO product for humans or animals.
4. EFSA acknowledges the fact that we have evidenced 40 significant differences on physiological parameters between rats eating GMOs and their controls. EFSA demonstrates in its counter-valuation of our study that what we have concluded is true: there is not 100% probability that all these 40 effects are only due to chance. Thus EFSA should conduct a very detailed toxicological analysis completely removed from their report on our study. By concluding that MON 863 is safe, EFSA accepts Monsanto reasoning on the fact that the effects are negligible since they are not proportional between the two doses (11 and 33% of GMO in the diet), and that they varied by sex. In our opinion these are significant departures from scientific principles. For instance, in the case of endocrine disruption, effects may not be proportional for two doses chosen arbitrarily a priori, and are rarely identical in males and females! Moreover there were signs of dysfunction (that we refer to as signs of toxicity) in liver and kidney that were different between rats fed the GMO diet and all other diets used in this experiment (6 diets in total, too many to study the GMO effect properly). EFSA has failed to consider the fact that the Monsanto study was not designed to observe a dose-response relationship (requiring more than two doses), that comparison of outcomes between GMO-fed rats and the entire variation of six reference groups is inappropriate, and that
endocrine differences by sex (effect modification) requires separate analyses.
5. EFSA discusses our weight curves for the animals at length, concluding that our comparisons were insufficient in accounting for individual variability. We note that we were the first to do this study that could have been performed by EFSA before. This demonstrates the lack of questioning of Monsanto tests by EFSA, since even in using methods that take a maximal account of individual variability, EFSA and the French Commission du Genie Moleculaire (CGB) still evidence some statistical effects on the variations of the female weights, as we do.
In conclusion, we appeal to the scientific community, government authorities and the public to question the EFSA scientific methodology in this case. Our recent paper stands as robust testimony to the questionable safety of this genetically modified food for humans and animals.
CRIIGEN
[NOTES]
1. EFSA reaffirms its risk assessment of genetically modified maize MON 863, Parma, June 28 2007.
http://www.efsa.europa.eu/etc/medialib/efsa/press_room/press_release/pr_efsa_maizemon863.Par.0001.File.dat/pr_efsa_mon863.pdf
2. New Analysis of a Rat Feeding Study with a Genetically Modified Maize Reveals Signs of Hepatorenal Toxicity by G.E. Seralini, D. Cellier & J. Spiroux de Vendomois, Arch. Environ. Contam. Toxicol. 52, 596?602 (2007).
EFSA fails to protect European citizens from a risky GM maize
http://www.greenpeace.org/eu-unit/press-centre/press-releases2/EFSA070628
Brussels, Belgium - The European Food Safety Authority (EFSA) missed an opportunity today to recover its credibility as regulator of GMO authorisations in Europe. The authority dismissed the call for further independent investigations into a Monsanto maize (MON863, already approved for sale in the EU), the subject of a scientific peer-reviewed study in March 2007 (1) which highlighted negative impacts suffered by rats fed with MON863 during feeding trials. The authors of the study warned that to disregard the signs of toxicity in the liver and kidney of the test animals would pose a danger to human and animal health.
EFSA's refusal to re-open the file on MON863 in the light of this study is consistent with the authority's stubborn refusal to assess GE applications in a balanced way: ever since it was established in 2002, EFSA has rubber-stamped every GMO application. It relies solely on data from agro-chemical companies, disregards long-term health and environmental impacts, and repeatedly dismisses divergent scientific opinions.
The timing of this announcement is telling. Having waited more than three months to release its opinion, it is no coincidence that EFSA chose to do so today, the very day that EU environment ministers are due to discuss the risk assessment of GMOs and the case of MON863 maize. Such an obvious attempt to influence a Council decision exposes the true motivations of EFSA, allegedly an objective agency of the European Union. At least now it is clear for all to see that EFSA is running a campaign to influence EU decisions and push risky GMOs on an unsuspecting public.
Two weeks ago, Greenpeace reported on a new study by French scientists showing similar threats of toxicity from another Monsanto GE maize (NK603) that the EFSA also recommended for sale in the EU, despite never having investigated disturbing anomalies in rats fed with the maize. Greenpeace is not alone in having criticised EFSA for failing to implement EU law while assessing the risks of GMOs. In April 2006, the European Commission issued a statement (2) calling for better test protocols and more research into the long-term effects of GMOs. The Council, too, repeatedly voiced concerns about EFSA's work. EFSA's recommendations on GMOs have never achieved formal backing by the required two-thirds majority of EU member states.
"Greenpeace is thus calling on EU member states and the EU Commission to reform EFSA's work to ensure that EU law governing GMO risk assessments is correctly implemented," said Marco Contiero of Greenpeace. "Until this reform is concluded, no further GE applications should be authorised." "The Commission should also withdraw authorisations already granted to other genetically-engineered products, given that they were approved under the same inadequate risk assessment procedure. The time has come for the EU to put the precautionary principle before the vested interests of agro-chemical companies such as Monsanto," he added.
EU environment ministers meeting today will discuss a proposal for further investigations into Monsanto's MON863 maize and the GMO risk assessment process.
Related Reports
EFSA's risk assessment on GMOs - 31 May 2006
Notes to Editor
1. Seralini, G.E., et al, 2007, 'New Analysis of a Rat Feeding Study with a Genetically Modified Maize Reveals Signs of Hepatorenal Toxicity', published in Archives of Environmental Contamination and Toxicology 52, 596-602
2. European Commission IP/06/498, 12 April 2006 http://europa.eu/rapid/pressReleasesAction.do?reference=IP/06/498&format=HTML&aged=1&language=EN&guiLanguage=en
Contact information
For technical background
Christoph Then, Greenpeace Germany GE campaigner, tel +49 171 878 0832
In Luxembourg
Marco Contiero, Greenpeace European Unit Policy Adviser on GMOs, tel +32 (0)2 274 1906
In Brussels
Katharine Mill, Greenpeace European Unit Media Officer, tel +32 (0)2 274 1903
SARGENT TO ACT QUICKLY ON ISSUE OF GM-FREE FOOD - The Irish Times, 27 June 2007. By Seán MacConnell.
The first moves to have Ireland move to where it can claim its food is produced without the aid of GM feed will be made soon by Minister for Food Trevor Sargent. On his first official engagement as Minister of State for Agriculture with responsibility for food and horticulture, Mr. Sargent said yesterday there was an urgency to move on the GM issue. "I have been getting reports from our markets in Italy and France that they are increasingly moving in the direction of requiring that produce be fed on GM-free feed," he said. Ireland does not have a clear position in my mind, as yet, on the direction we are going in that regard," he said at the launch of the latest Bridgestone Guide. "I want to bring together the farming organisations, the food retailers, the grain importers and the people in the Department of Agriculture so we can formulate a strategy in the best interests of the producers and the country." He said countries which were already able to make this claim were threatening Irish exports and using GM-free status as a marketing tool.
He addressed issues raised by the joint editor of the Bridgestone guide, John McKenna, who sought changes in the regulations governing farmers markets and specialist portable abbatoirs for organic producers. Mr Sargent said he would be working with the Minister for the Environment, John Gormley, to bring about some standardisation in the regulations covering farmers markets. He said he wanted to build a bridge between large-scale producers and the farmers' market system which would allow them to produce for the multiples and for the local markets. There were not enough local abbatoirs and this was holding back the development of distinctive artisan food production, particularly in the organic sector and he would be looking at the mobile abbatoir system being used on the Continent.
Doctors obliged to oppose genetically modified foods - 15 June 2007 - By Greg Baxter
http://www.imt.ie/displayarticle.asp?AID=13118&NS=1&CAT=18&SID=1 <http://www.imt.ie/displayarticle.asp?AID=13118&NS=1&CAT=18&SID=1
Doctors have an ethical and moral duty to oppose genetically modified (GM) foods in the interest of the health of future and present generations, the Secretary of the Irish Doctors Environmental Association (IDEA) has stated. Dr Elizabeth Cullen, a public health doctor in Kildare, has said in a paper set to be published in the Irish Medical Journal: "If planting of genetically engineered crops is allowed in Ireland, we will leave an irreversible legacy to future generations... permission to grow or consume genetically engineered foods in Ireland should be denied."
IDEA is partially sponsoring a major conference on GM foods in Dublin on 15 June, entitled: Is the European Food Safety Authority downplaying the health risks of genetically modified food The conference, which has been organised by GM-Free Ireland, will hear international speakers discuss the health risks of GM foods, as well as the process of genome scrambling, GM maize, and the European Food Safety Authority.
Greece Extends Biotech Ban - The Associated Press, June 26 2007 - http://www.chron.com/disp/story.mpl/ap/fn/4921613.html
ATHENS, Greece - Greece on Tuesday extended for two more years a ban on genetically modified maize seed developed by U.S. biotech giant Monsanto. A decision also expanded the sale-and-cultivation ban from 31 to 51 varieties of the MON810 seed type, Deputy Agriculture Minister Alexandros Kondos said. "The ministry strongly opposes the circulation of genetically modified organisms," Kondos said. "Our target is to produce quality products, and under no circumstances do modified products qualify as such."
Despite pressure from the European Union, Greece has implemented and extended bans on the MON810 strain since April 2005. This month, EU Trade Commissioner Peter Mandelson warned that member states could invite legal action against the bloc from the World Trade Organization by adopting individual bans on biotech food instead of deciding on common rules.
But Greece insisted its action was well-advised. "(The new ban) is founded on the same solid scientific and legal basis, but also includes new scientific data and finds," an Agriculture Ministry statement said. "These concern a possible threat to human health, and well as to the beekeeping industry." It said EU member-states "need to be granted a satisfactory time frame to assess the dangers from growing genetically modified strains."
Monsanto maize approved for human consumption potentially toxic, warns new study
Greenpeace demands immediate withdrawal of suspect maize from the market and review of regulatory system
http://www.greenpeace.org/eu-unit/press-centre/press-releases2/seralini-NK603
Brussels, Belgium - New research into the health impacts of genetically engineered (GE) food already approved in Europe casts further doubt on the way these products are checked for safety by EU authorities before being approved for sale and consumption. The study, carried out by French scientific research institute CRIIGEN on the results of rat feeding trials using a GE maize made by biotech firm Monsanto, highlights 60 significant differences between the rats that were fed the GE maize and those fed normal maize (all for 90 days). The first group showed differences in their kidney, brain, heart and liver measurements, as well as significant weight differences. These could be warning signs of toxicity, but have not been further investigated.
"Greenpeace is deeply concerned that genetically engineered crops and foods are getting the EU green light for sale despite alarming health anomalies occurring in test animals over very short test periods. We will be faced with eating these foods for years," said Marco Contiero policy adviser on GMOs at Greenpeace European Unit.
The Monsanto maize, known as NK603 maize, has been engineered to tolerate Monsanto's own herbicide. Approved for import for use in human food and animal feed in 2004, it is currently being tested for cultivation in field trials in Europe.
The scientists at CRIIGEN analysed Monsanto's own test results, which had informed the EU food safety authority's decision to approve the maize for sale. CRIIGEN's report [1] suggests that a far more thorough investigation is necessary. Neither Monsanto nor the scientific committees consulted on the feeding trials disputed the differences found in the test animals compared to the control group. However, they dismissed the results as "not of biological significance". The CRIIGEN study questions that conclusion.
"It is alarming that a company which produces a genetically-engineered crop not only gets to design and conduct the safety tests of its own product, but also to analyse the results. The lack of any independent scrutiny of test data suggests that Europe's risk assessment procedure is overlooking the threats and not assessing risks at all, just rubberstamping company dossiers," said Marco Contiero.
This is the second such case: another Monsanto maize, known as MON863, subject of a peer-reviewed scientific study published in March 2007, also showed signs of toxicity of liver and kidney in rats fed with this maize over a period of three months.
Professor Gilles-Eric Seralini of CRIIGEN, the University of Caen and the French state commission on biotechnology (Commission du Genie Biomoleculaire, CGB) said: "The statistical analysis should be repeated by independent experts and the crude data put on a website for the scientific community to be involved. Further testing should always be carried out if the analyses of the data do not result in a clear outcome."
Greenpeace is campaigning for the withdrawal from the market of NK603 maize, pending further investigation and a re-evaluation of Monsanto's trials, and for the suspension of all genetically engineered product and crop authorisations until the current EU risk assessment system is reviewed.
Contact information
Katharine Mill, Media Officer, Greenpeace European Unit - katharine.mill@diala.greenpeace.org - Telephone: +32 2 274 1903
Marco Contiero, Greenpeace European Unit, Policy Director - GMOs - marco.contiero@diala.greenpeace.org - Telephone: +32 2 274 1906
See the report
EU stands up to US pressure – unfazed by genetically modified ‘Herculex’
Brussels, June 25 2007 - EU member states today stood up to intense pressure from the US and refused to allow a new strain of genetically modified maize to be imported into the EU. The move has been welcomed by Friends of the Earth Europe. [1]
European Commission documents show US pressure to ignore risk assessment concerns and push GMOs - including this GM Maize ‘Herculex’ of biotech company Pioneer - onto the European market [2] [3].
Helen Holder Friends of the Earth Europe GM Campaign Coordinator said: “Member states have already won the right to uphold high standards on food safety and the environment at the WTO. The US had tried to use trade laws to force GMOs into the European market. But this is a clear signal that Member States have put safety and the environment before US trade interests and that the concerns of EU citizens can prevail over formidable lobbying from biotech companies”
Friends of the Earth Europe and other NGOs have raised a number of concerns on this GM maize:
* The risk assessment was incomplete and failed to act on key evidence which raised the possibility that this GM maize could pose risks for human and animal health [4]
* The reliability of EFSA opinions on other related GM maize has been undermined by studies of independent scientists detecting toxicological effects in the same products. [5]
* Herculex Maize has been at the centre of a number of contamination scandals including the contamination of US animal feed imported
into Ireland. [6] The sudden and rapid move to try and authorize Herculex maize suggests that the European Commission is more concerned with ‘neutralizing’ a potential legal problem of illegal GM contamination rather than dealing with contamination by unauthorized GMOs.
Helen Holder added, “These contamination cases indicate more than ever before just how important it is to show zero tolerance to countries that have lax measures on contamination and to ensure the right to GMO free food and farming in the EU is upheld. There is a critical need for strict laws on growing GM crops and clear rules on who is liable for the costs of GM contamination.”
There is still widespread public concern over the loophole in EU legislation that allows for consumers to remain unaware that they are eating meat and dairy products from animals fed with GMOs like Herculex maize. Earlier this year one million Europeans called for labelling of foods from GMO-fed animals.
[1] The vote resulted in a Non Qualified Majority, insufficient votes to reach a decision. The copmpany's authorisation request will now be sent an upcoming EU Council meeting where Ministers will vote on Herculex.
[2] Herculex Rootworm (RW) 59122 maize has been genetically modified to produce Bt toxins (Cry34Ab1 and Cry35Ab1) in order to be resistant to the Western corn rootworm insect pest.
[3] Minutes of a meeting between the EU and the US were obtained by Friends of the Earth Europe under a Freedom of Information request:
http://www.foeeurope.org/press/2007/May30_HH_EU_US_docs.htm
[4] The studies by Pioneer/Dow submitted to the EU show important differences between animals fed with GM maize and those fed with conventional maize, including liver weights in females in a 42 day poultry study, and blood parameters following a 90 day rat feeding trial. Effects in the 90 day feeding trial were noticed after a very short time, indicating potential for toxicity in the longer term.
[5] The EFSA has in the past issued positive opinions on MON863 and NK603 maize, leading to final authorization by the European Commission of these products. But the reliability of these EFSA opinions has been undermined by recent studies by independent scientists showing toxicological effects in both MON863 and NK60.
[6] Announced by Greenpeace and GM-free Ireland. See GM-free Ireland press release http://www.gmfreeireland.org/pakrac/index.php
Cyprus to Shelve GM Food Separately - The Associated Press, June 15 2007
http://www.chron.com/disp/story.mpl/ap/fn/4893405.html
NICOSIA, Cyprus - Cyprus' parliament has passed legislation requiring supermarkets to put genetically modified products on separate shelves from other food. The Green Party, which tabled the bill, said Friday that the legislation will make it easier for the public to "distinguish these products" and help them make more informed purchases. Parliament unanimously approved the bill Thursday.
The Green party described the bill as a "historic victory" that would lead the biotech food industry to abandon the Cypriot market. Party spokeswoman Ioanna Panayiotou said surveys have shown wide public support for the initiative - which she said was the first of its kind in the European Union. "It is a pioneering moment ... and we are optimistic more countries will follow," she told state radio.
IRELAND AIMS TO BECOME A GMO-FREE ZONE - PRESS RELEASE
Dublin, Thursday 14 June 2007 - GM-free Ireland Network - www.gmfreeireland.org
New coalition government adopts all-island GM-free policy - Farming groups agree to explore phasing out GM animal feed
DUBLIN, 14 June 2007 Following last night?s Green Party historic agreement to form a coalition government with Fianna Fáil, the two parties revealed their agreed policy "to negotiate for the whole island of Ireland to become a GMO-free zone."
The announcement was received with jubilation by farmers and food producers on both sides of the border who have spent the last nine years campaigning to achieve this goal. Following the announcement last night, Green Party leader Trevor Sargent, TD, confirmed his pre-election pledge to resign his leadership position if his party entered into government with Fianna Fáil, adding that he would now work very hard within the new Government "to get Green Party policies implemented whatever way I can".
"The establishment of Ireland as a GMO-free zone is a project that I will throw myself into in a very enthusiastic fashion, because we don't have much time to rescue that status for this country, and it's one that is so vital to us as a food-producing island which is operating in markets that are overwhelmingly looking for GM-free food, and if we lose that status, that?s it, we cannot go back."
The Green Party is an all-island party, working on both sides of the border. The Green Party will get two Cabinet Minister positions in the new Government.
In a related move last weekend, the GM-free Ireland Network brought the main farmers organisations on both sides of the border together with Brazil?s largest exporter of certified non-GMO soya beans, for exploratory discussions to phase out the use of GM animal feed in Irish farming. Participants included high level representatives of the Irish Farmers Association, the Irish Creamery and Milk Suppliers Association, the Irish Cattle and Sheepfarmers Association, and the Northern Ireland branch of the UK National Beef Association.
Measures and actions to protect Cyprus from GMOs
FEDERATION OF ENVIRONMENTAL AND ECOLOGICAL ORGANISATIONS OF CYPRUS
28 Athalassis Av. aprt. 12, 2012 Strovolos, Nicosia. Tel: ++357 22 313750 Fax: ++357 22 879241
email: info@oikologiafeeo.org website: http://www.oikologiafeeo.org/
The Minister of Agriculture and the Environment has organised a meeting with all relatedted stakeholders to discuss measures and actions to protect nature and human health from GMOs. The meeting took place on Monday 4th of June. The Minister has invited all the political parties, the farmers' organisations, the Federation of Environmental Organisations of Cyprus, Environmental Foundations, all related governmental Departments, the Consumers association, the Organic Farmers Association, the Union of Municipalities, the Union of Villages, the National Bioethics Committee, the Cyprus Employers and Industrialists Federation (OEB), the Cyprus Chamber of Commerce and Industry (KEBE) and the Attorney General.
The purpose of the meeting was to examine measures and actions in order to protect nature and public health from GMOs. All participants pressed for GMO free Cyprus, even the Governmental departments, apart from OEB, KEBE and the representative of the Attorney General. Hesitation was expressed from the representative of the Attorney General, who made it clear that a total ban is in inconsistency with the acquis. The OEB and KEBE considered the EU directives adequate to protect from GMO effects, but they didn't express opposition to ban GMOs cultivation in Cyprus. They wouldn't agree on total ban due to trade barriers.
It has been decided:
* to carry out a study to document that coexistence in Cyprus is impossible
* to investigate ways for declaring Cyprus to a GMO free area, in the frame of the acquis
* to examine the possibilities for stricter provisions in the existing laws on GMOs in the frame of the acquis
* to find ways protecting Cyprus agriculture and wild species from adventitious contamination due to animal feed
* to look for allies member states in order to promote changes in the EU laws regarding GMOs
* better staffing of the responsible governmental departments and intensification of the tests on GMOs
* stricter implementation of the related legislation
* to support Parliaments plans for new Law on separating genetically modified food products on supermarket shelves
* to promote public awareness on the issue
Please note that the Cyprus government will look for allies. It is considered as a very important motion to press Commission.
The anti GMO Committee Of the Federation of Environmental and Ecological Organisations of Cyprus
More EU states wary on GMO maize - By Jeremy Smith - SCIENCE NEWS - Reuters News Service, June 5 2007
http://www.sciam.com/article.cfm?alias=more-eu-states-wary-on-gm&chanID=sa003&modsrc=reuters
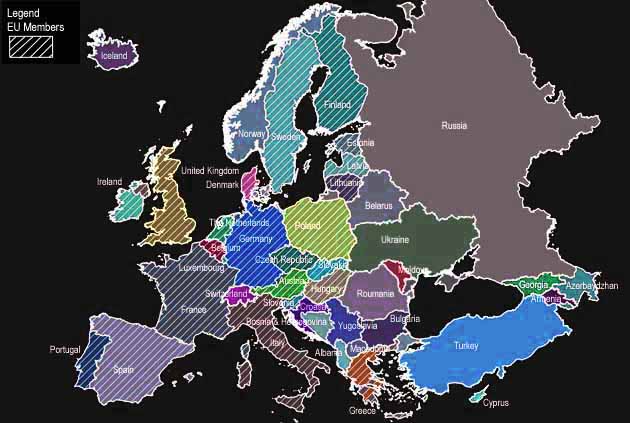
BRUSSELS (Reuters) - Several influential EU states have dug in their heels on whether their farmers may grow one of Europe's oldest genetically modified (GMO) crops, raising the stakes in the EU's long-running stalemate over biotech policy. The crop is a modified maize variety known as MON 810, marketed by leading U.S. biotech seeds company Monsanto. Also known by its commercial name YieldGard, the maize type is designed to resist the European corn borer, a pest that attacks maize stalks and thrives in warmer climates in southern EU countries such as Italy and Spain. While Monsanto says the protein contained in its maize has selective toxicity but is harmless to humans, fish and wildlife, an increasing number of the EU's 27 countries are unconvinced.
National GMO bans are the only part of Europe's biotech debate where EU countries can agree, since they see attempts by Brussels to order a government to lift its ban as an attack on national sovereignty. So, unusually, they tend to band together. The European Commission has tried this on three occasions in the past two years and got a stinging rebuff on each occasion. In the past few weeks, two EU agricultural powerhouse countries - France and Germany - entered the fray. Not only do they wield huge clout under the bloc's weighted voting system for decision-making, they also grow vast amounts of cereals. First, Germany's government said maize produced from MON 810 seeds could only be sold if there was an accompanying monitoring plan to research its effects on the environment: a restriction that farmers say is tantamount to a growing ban. The proposed restriction, to apply from 2008, has already been notified to Commission authorities in Brussels. Soon afterwards, French government number two Alain Juppe, in charge of his country's environment, transport and energy policy, said in a newspaper interview that he would not exclude being "inspired" by Germany's proposed GMO ban.
Diplomats said it was too early to know if the French and German stances would affect voting for new GMO approvals - EU countries have clashed over this for years - but warned that it might alter the balance between 'pro' and 'anti' GMO countries. "Even a national ban would get them into hot water with the Commission, but if it's a blanket change in position (on biotech policy) then it raises the stakes," one said.
NATIONAL BANS
Austria banned MON 810 maize in June 1999, around 14 months after the EU issued its original authorization. That national ban was cited, along with several others, by Argentina, Canada and the United States in an international challenge against the European Union at the World Trade Organization a few years ago. Hungary, one of the EU-27's biggest grain producers, became the first eastern European country to ban GMO crops or foods when it outlawed the planting of MON 810 seeds in January 2005. The same year, Greece and Poland used a provision in EU law that allows countries to decide whether to allow GMO seeds on national territory - although a ban must be approved by EU member states to be legal. Both countries have restrictions in place against MON 810 maize. Bulgaria's parliament has also indicated support for national restrictions for growing MON 810 maize.
EU environment ministers have slapped down several draft orders authored by the European Commission for countries to rescind their national GMO bans. This happened last February in the case of Hungary and also in December 2006, for Austria. "We have had two councils (EU ministerial meetings) that have rejected Commission proposals (to lift GMO bans) with a large majority, and now there is this additional case in Germany," a Commission official told Reuters. "We have to look at the whole (GMO) authorization policy at some point."
US still bullying EU to market GMOs - But avoid the dirty GMO word! advises US official
http://www.foeeurope.org/press/2007/May30_HH_EU_US_docs.htm
30 May 2007, Brussels - New documents obtained by Friends of the Earth reveal that the United States continues to pressure the EU to market new genetically modified (GM) crops and foods, despite the World Trade Organisation's recent verdict that the EU has a right to protect itself against GMOs. In an email exchange, US officials even insisted that the EU should steer clear of the term "GMOs" in order to minimize public opposition to its policies.
Friends of the Earth Europe GMO Campaign Coordinator Helen Holder said: "Even after they failed to win at the WTO, the US, and their friends in the biotech industry are still trying to force feed European citizens GMOs. The European Commission must stand firm, and put European citizens' health, the environment and the right to GM-free food and farming before the interests of a few big corporations."
The documents - email correspondence and minutes of a meeting between the European Commission and the US earlier this year - were obtained by Friends of the Earth Europe under a freedom of information request. The documents reveal US frustrations at the EU's failure to "normalize trade" of agricultural biotechnology products and at the "lack of political will to operate EU approval systems of GMOs" due to member states' opposition.
The US was pushing the European Commission to:
*Ignore risk assessment concerns and push GMOs quickly on to the EU market
*Agree a deal to fast-track GMOs that the US wants to be sold in Europe
*Authorize a controversial genetically modified oilseed rape [canola] as proof that the Commission is backing down under US pressure
*Bring top EU decision makers into line with US policy and commit to allowing GMOs into the EU
*Abolish member states' national bans
*Lower standards for GM contamination of food for GMOs that are not authorized in the EU
The World Trade Organisation (WTO) issued its final ruling of the GMO dispute last autumn, finding no clear winner or losers. It did not question the right of counties to put in place strict biosafety laws, nor the right of a country to ban an individual GMO. The GMO bans in place when the complaint was lodged were ruled illegal on a technicality only [3].
For more information, please contact:
Rosemary Hall, Communications Officer at Friends of the Earth Europe: Mobile +32 485 930515, rosemary.hall@foeeurope.org
Helen Holder, coordinator of the Friends of the Earth Europe GMOs campaign: Mobile +32 474 857638, helen.holder@foeeurope.org
For further background see FoEE media briefing: http://www.foeeurope.org/publications/2007/FoEE_GMOS_US_pressure_on_EU_brief_May07.pdf
[1] http://www.foeeurope.org/GMOs/2007/Annex1_US_EC_Emails.pdf
[2] http://www.foeeurope.org/publications/2007/FoEE_GMOS_US_pressure_on_EU_brief_May07.pdf
[3] For an overview of the WTO dispute ruling see: http://www.foeeurope.org/publications/2006/WTO_briefing.pdf
Friends of the Earth Europe campaigns for sustainable and fair societies and for the protection of the environment, unites more than 30 national organisations with thousands of local groups and is part of the world's largest grassroots environmental network, Friends of the Earth International.
France could follow Germany in GM corn restriction - http://www.expatica.com/actual/article.asp?subchannel_id=25&story_id=40199
PARIS, May 25, 2007 (AFP) - France may follow Germany in imposing restrictions on a strain of genetically-modified corn made by the US giant Monsanto, Ecology Minister Alan Juppe was quoted Friday as saying. "Germany has just suspended authorisation for MON 810 seed. In this particular instance, we must be steered by the German case," the newly-appointed minister told the daily Le Parisien. "They have just discovered that the toxin which is supposed to kill the corn pest is being secreted in ways that are not precisely what was expected." MON 810 is a genetically-engineered breed of corn, also called maize, that
exudes a poison to kill an insect called the European corn-borer.
Juppe was referring to restrictions applied by the German federal government, which has reportedly told Monsanto that MON 810 may only be sold if the firm also provides an accompanying "monitoring plan" to research the effects on the environment. The restrictions have the impact of a de-facto moratorium, as Monsanto has not presented any MON 810 monitoring plan so far. Juppe said, however, that transgenic research, both in medicine and in food, "should not be stopped".
Genetically-modified foods, while widely on sale in North America, are a deeply sensitive issue in Europe, where green groups are fighting to have them banned. Juppe said the question of GM crops in France would be addressed at a national review of environment policies, expected to be held in October.
Copyright AFP
MEPs CALL ON THE COMMISSION TO BAN THE INTRODUCTION OF GMOs - Biodiversity conservation plan 'insufficient', say MEPs
EUactiv.com, 23 May 2007 [shortened]
http://www.euractiv.com/en/environment/biodiversity-conservation-plan-insufficient-meps/article-163937
Halting the decline in biodiversity will require "unprecedented efforts", according to [the European] Parliament...
Related Documents:
Halting biodiversity loss by 2010 - an EU action plan
http://www.euractiv.com/en/environment/halting-biodiversity-loss-2010-eu-action-plan/article-157424
With just three years to go until the 2010 target date for halting the decline in biodiversity set by European heads of state in 2001, MEPs voiced their "profound concern at the continuing loss of biodiversity", in an own-initiative Report adopted on 22 May. Parliament considers the Commission's 2006 Action Plan to be "insufficient to conserve biodiversity and sustain ecosystem services in the longer term". Rapporteur Adamos Adamou's report demands that the Natura 2000 programme be strengthened in order to restore species, as well as safeguard them.
It also calls for a Community response to the threat posed by the introduction of "invasive alien species and alien genotypes", suggesting that immuno-contraception could have a decisive role to play. MEPs call on the Commission to ban the introduction of genetically modified organisms and evaluate the potential threat to biodiversity posed by their introduction.
The Commission welcomed the report, sharing Parliament's strong concern at financial constraints to implementation and at the continuing loss of biodiversity and the related decline of ecosystems. It supports Parliament's position that the maintenance of ecosystems should become a fundamental goal of all EU horizontal and sectoral policies.
CRACKDOWN ON MONSANTO SEEDS - Germany Tightens Restrictions on Genetically Modified Corn - Der Spiegel, May 9 2007
http://www.spiegel.de/international/business/0,1518,481952,00.html
The German government has imposed stricter regulations on the food company Monsanto regarding the sale of genetically modified corn seeds. The new rules are tantamount to an outright ban. Genetically modified (GM) crops have long been controversial in Germany, where organic agriculture is booming. Now the cultivation of GM corn has been effectively banned by the government, according to media reports.
In its Wednesday edition, the Berlin daily Der Tagesspiegel reports that it has obtained a letter sent from the Federal Ministry of Agriculture to the agricultural company Monsanto, which sells the GM corn MON 810 -- which has been legal in Germany up until now -- as seed. In the letter, the ministry writes that GM corn from the MON 810 product line can only be delivered to third parties if the firm also provides an accompanying monitoring plan which researches the effects on the environment. The German news agency DPA also reported Wednesday they had obtained a copy of the same letter.
"This amounts to a de facto ban on the cultivation of genetically modified corn," said Peter Rudolph, who is responsible for genetic technology in the Brandenburg state ministry of agriculture, in remarks to Der Tagesspiegel Tuesday. He said the letter basically means Monsanto will no longer be allowed to sell MON 810, as the company has not presented any monitoring plan up until now. Brandenburg is the German state with the largest quantity of GM corn under cultivation.
In the letter, the federal ministry justifies its decision by writing that new information "gives reasons to suppose that the cultivation of MON 810 poses a danger to the environment." A spokesperson for the Ministry of Agriculture told the newspaper that the letter should not be interpreted as a ban, but rather as a tightening of the regulations concerning the cultivation of the GM corn. The new ruling could mean that crops already planted may not be allowed to be harvested. Brandenburg farmer Jorg Piprek told Der Tagesspiegel the new ruling was absurd: "We've already planted the corn. They can't tell us after the fact that it was illegal."
The cultivation of genetically modified crops has been controversial all over Europe, with anti-GM activists going as far as ripping up crops. The German Agriculture Minister Horst Seehofer has up until now justified the cultivation of GM crops in Germany by arguing they are allowed under European Union regulations.
Netherlands refuses GM corn shipment from US - Agence France-Press (AFP), May 9 2007
http://www.france24.com/france24Public/en/administration/afp-news.html?id=070509193328.8sqb1a72&cat=science
The Netherlands will return, or burn, a United States shipment of genetically modified corn that lacks clearance from European authorities, the Dutch food security authority said Wednesday. Environmental organisation Greenpeace recently denounced the corn gluten shipment, which arrived at the Rotterdam port on April 10. The genetically modified corn was not authorised by the European Union, the Dutch authority said in a statement. The name of the product in the shipment was Herculex RW from US company Pioneer, the authority said. It is used in the production of animal feed. The portion of the shipment not yet used will be recalled and either returned to the United States or burnt in the Netherlands, according to the authority. The product is currently being tested by the European food safety agency, with EU experts set to decide on June 8 whether it will be authorised. For that reason, Dutch authorities did not order the destruction of products that have already incorporated it. Food safety authorities in the Netherlands have also decided to increase testing for genetically modified foods. From now on, 25 percent of corn imports from the United States will be tested, compared to the previous 10 percent.
ILLEGAL GM MAIZE ENTERS EU THROUGH IRELAND - http://www.gmfreeireland.org/pakrac/index.php
*Greenpeace and GM-free Ireland demand blockade of all US maize shipments
*Clouds of GM powder contaminate Dublin Docklands
*Irish farmers move to phase out use of GM animal feed
Download press release - http://www.gmfreeireland.org/press/GMFI35.pdf
Download high-res versions of the photos on this page - http://www.gmfreeireland.org/pakrac/index.php
Briefing on illegal GM maize available upon request
PERSON TO CONTACT - Michael O'Callaghan, Co-ordinator, GM-free Ireland Network - tel + 353 (0) 404 43 885 - mobile: + 353 (0) 87 799 4761
email: mail@gmfreeireland.org - www.gmfreeireland.org
German group denied permit to grow GM rapeseed in Lithuania - RIA Novosti, April 4 2007 - http://en.rian.ru/world/20070404/63086603.html
Lithuanian authorities reaffirmed their opposition to the spread of genetically modified crops Wednesday by rejecting an application from a German company seeking to grow GM rapeseed in Lithuania for research purposes. The Environment Ministry said in a press release that it had taken public opinion into account when making the decision, as well as the views of the scientific community. According to a survey conducted by the Fonitel agency, 63% of Lithuanians are opposed to the cultivation of genetically modified crops in their country. Last year, Lithuania rejected a similar application for growing GM potatoes.
LAW ON FOOD (Croatia): HSP Against Importing GMO Products - Jelena Simac - Javno.com, April 3 2007
http://www.javno.com/en/croatia/clanak.php?id=32277
HSP [Croatian political party] is against the proposed law concerning food, which would allow the import of genetically modified seeds and food for animals. At a press conference today, HSP requested the withdrawal of the Cabinet's proposed law, which would allow the import of genetically modified seeds and food for animals. The law, as it was said, was supposed to be voted in an urgent session, and was make according to the recommendations of the European Commission. HSP thinks that the import of such food would be very harmful for Croatia. "Croatian agriculture is dead if we start to import GMO. Our country is competitive only because it possesses ecologically clean products, and because of the low amount of artificial fertilizers and pesticides." said HSP's Miroslav Rozic.
HSP is still concerned about the last article of the law, with which the previously accepted law ceases to exist. Removing that law, Croatia would be directly exposed to great imports of genetically modified products without any restrictions. "Instead of the proposed law going through a detailed public discussion, it must be brought through an urgent procedure" said Rozic, with the assumption that Croatia committed itself to the European Commission and the law must be accepted by the end of March. "Through this the hypocrisy of the European Union can be seen. HSP hopes that it will not lose the war against GMO" added Rozic.
According to the thoughts of HSP, the disputed proposed law is "literally a half translated directive of the EU, with English syntax", and its parts have nothing to do with Croatian reality.
Russia joins the battle over GM products - FreshPlaza, March 07, 2007
http://www.checkbiotech.org/root/index.cfm?fuseaction=news&doc_id=14583&start=1&control=206&page_start=1&page_nr=101&pg=1
Moscow - On July 1, the city of Moscow will introduce a voluntary system of food labels indicating that a product does not contain genetically modified (GM) ingredients. Europe has recently been engaged in a battle with the World Trade Organization (WTO), which, taking its cue from the US, Canada and Argentina, considers the European Union's moratorium on GM products illegal. Meanwhile, Europeans have been collecting signatures and protesting against GM foods. In the US, a lawsuit was filed against the Department of Agriculture after it legalised the commercial production of GM alfalfa sprouts. The court found the agency's actions illegal.
In 2000, 828 scientists from 84 countries signed an open letter to the world's governments warning them of the hazards of GM foods. Environmental organisations demanded that the UN declare a moratorium on GM products. Arguments in favour of GM foods - high crop yields, resistance to diseases, insects and harsh weather, and their low price (they tend to cost 20-30 percent less than traditional foods) - have also been widely challenged, though without hard evidence. Environmentalists say that GM foods will not solve the problem of world hunger, but they will bankrupt small farmers. Some biologists believe that GM foods can have a negative effect on the gene pool and reduce biological diversity. Vladimir Kuznetsov, head of the Institute of Plant Physiology at the Russian Academy of Sciences, said that GM foods are dangerous because they are unpredictable. 'Scientists do not know what effect they will have on the human body in the long term,' he said.
Research is being conducted on GM foods' effects on human health, particularly those that may trigger allergic reactions, but not all of the results have been made public. There is much debate but few facts. One thing is certain: the GM industry will continue to grow. But how much? In January, at the Council on Human Rights Policy in the Kremlin, Natalya Olefirenko, a Greenpeace Russia representative, said that in most Russian regions GM products account for 10-20 percent of the market. In some cities without sufficient controls in place, the figure is 50 percent. In recent years, imports of GM foods (Russia does not produce them) have increased by more than 100 times. The main GM crops are soy beans, potatoes, corn, sugar beets and oilseed rape. By law, products that contain more than 0.9 percent GM ingredients must be labelled, but in practice this rule is often ignored.
Meanwhile, according to an All-Russia Public Opinion Research Center poll, 95 percent of Russians who have heard of GM foods would not buy them if the products were labelled as such. Consumers, however, are still not able to exercise their right to choose. Russian bio-engineers, among them Konstantin Skryabin, director of the Bio-Engineering Center at the Russian Academy of Sciences, believe that GM foods "won't get out of the laboratory until they are thoroughly tested. Meanwhile, Russia, with its non-competitive agricultural market, has to move faster to grow and popularise GM crops." He added that the GM issue has more to do with business than with science.
Russia's President Vladimir Putin holds a different view. 'With our entry into the WTO, certain issues have to be addressed," he said in January. "American and Canadian products, which are, as a rule, genetically modified, are competing on the world agricultural market." He added that "we can use Europe's experience" and "we must inform people about the hazards of GM products." Putin proposed to set up a council to regulate GM food. Europe uses diverse methods to combat GM products, including the destruction of genetically modified crop fields in France, passing laws that limit the possibilities of growing GM crops in Germany, banning GM versions of local and protected crops in Bulgaria, or banning all GM products in Poland. The end result of all this is the creation of GM-free zones. Russia is following Europe's example. The city of Moscow and the Belgorod Region are leaders in this process. The idea of creating GM-free zones is being discussed in the Volgograd, Kostroma, Murmansk, Ryazan, Sverdlovsk and Ulyanovsk regions.
Moscow's law stipulates that all agricultural raw materials or food that is brought to the city through an organised supply system must contain information about their GM ingredients. It is illegal to use budgetary funds to buy GM children's food. Following an inspection of their food, producers will have the right, valid for one year, to put the label 'This product does not contain genetically modified ingredients' on any kind of product. As much as 50 million rubles ($1.9 million) will be set aside to purchase special equipment. The media will inform the public about producers that sell GM products but do not tell consumers. So while bio-engineers complain about a campaign to discredit GM foods, their opponents are demanding a moratorium to give researchers time to study their medical and biological effects. In the meantime, consumers are trying to make sense of all that is being said about GM foods.
EFSA to review Monsanto maize concerns - By Stephen Daniells - GoodNavigator.com, 15 March 2007
http://www.ap-foodtechnology.com/news/ng.asp?n=74981-monsanto-efsa-mon-toxicity-gm
15/03/2007 - The European Food Safety Authority (EFSA) has revealed that it will review the new data presented by French scientists that revealed toxicity concerns in rats fed the MON863 variety of GM maize from Monsanto. The new data, from a 90-day rat study and published in the peer-review journal Archives of Environmental Contamination and Toxicology, indicated liver and kidney toxicity in the rats, as well as differences in weight gain between the sexes as a result of eating the transgenic maize. Alun Jones, EFSA spokesperson told FoodNavigator.com that the authority has not yet had the opportunity to look at the new study in detail but this will be done by their scientific experts before any decisions is made regarding the maize. The GMO panel will meet on March 22nd and 23rd to consider and discuss the new study.
Jones also stated that this was not the first time that EFSA have been requested to look at MON863. Indeed, the authority released a statement in October 2004 following a request by the German authorities following a 13-week rat study that suggested kidney toxicity. "Following [the GMO Panel's] investigation of the report, and of the retrospective evaluation of renal tissues and data derived from the 13-week rat feeding study performed by independent peer reviewers, the GMO Panel concludes that there is no evidence presented in the report that changes the conclusions already reached by the GMO Panel earlier this year in its Opinions on the safety of the insect-protected genetically modified maize MON 863 (EFSA 2004a, b)," read the October 2004 statement. "These opinions state that the results of the rodent toxicity study with MON 863 maize did not indicate concerns about its safety for human and animal consumption."
The researchers behind the new study, led by Professor Gilles Eric Séralini from the independent CRIIGEN (Committee for Independent Research and Genetic Engineering) based at the University of Caen questioned the methods used by Monsanto to initially show the safety and non-toxicity of the corn, saying that the statistical methods used were insufficient to observed any possible disruptions in biochemistry. "Monsanto's analyses do not stand up to rigorous scrutiny - to begin with, their statistical protocols are highly questionable. Worse, the company failed to run a sufficient analysis of the differences in animal weight. Crucial data from urine tests were concealed in the company's own publications," said Séralini during a joint press conference with environmental group Greenpeace in Berlin.
Monsanto have continued to defend the safety record of their corn. Spokesperson, Lee Quarles, told FoodNavigator.com: "The important thing to note in all of this is the fact that the overwhelming opinion of expert authorities is that MON 863 is safe for human and animal consumption. This includes experts in Europe as the European competent authorities concur that MON 863 YieldGard Rootworm maize is safe for human and animal health and the environment. "Please also note that MON 863 YieldGard Rootworm maize has completed full regulatory review and has been grown commercially in the United States and Canada since 2003. This product has also been approved for import and food use in many countries around the world, including Japan, Korea, Taiwan, the Philippines, Russia and Mexico," he added.
MON863 is a transgenic maize genetically modified to express the Bt-toxin (Cry3Bb1) which enables the plant to be insect repellent against the corn rootworm pest. It is different from other GM corns of the market since these express the Cry1Ab toxin which is toxic to the European corn borer. It received European approval for use in animal feed in 2005 and for human consumption in 2006.
French Scientists Express Doubt About Genetically Modified Corn - DeutscheWelle, 13 March 2007
http://www.dw-world.de/dw/article/0,2144,2382626,00.html
The environmental protection organization Greenpeace has long said genetically modified maize could be a health hazard. Now, in a new study, a group of French scientists have also expressed their doubts about the corn. Greenpeace has warned about the potential dangers of genetically modified (GM) produce and maize for some time. On Tuesday they presented a study in Berlin to backup their claims. Professor Gilles-Eric Seralini of the University of Caen said that according to studies by his group, CRIIGEN, Monsanto's maize type MON863 caused symptoms of poisoning and liver and kidney damage in rats that were fed the product during experiments. Seralini's results call into question an earlier report by Monsanto that said genetically modified feed was harmless. "There are significant deficits in the statistic evaluation of the Monsanto report," Seralini said. Genetically altered maize could therefore not be deemed safe, Seralini said.
Greenpeace genetic engineering expert Christoph Then said the case shows that "German Consumer Affairs Minister Horst Seehofer must stop the sowing of GM seeds and the import of GM food in Germany."
Built in pest control
MON863 has been cultivated since 2003 in several countries, including the United States and Canada. The GM maize, which can be legally imported into European Union countries since 2006 as a food and feed product, contains a protein to combat plant pests, allowing farmers largely to grow their maize crops without having to use pesticides. Seralini, however, said he found that GM maize produced around one kilogram of poisonous substances per hectare. He said that is more than farmers would use in pesticides. The scientist also pointed out that Monsanto ran tests with animals fed with MON863 for only 90 days. Long-term studies do not exist, he said.
As safe as unmodified corn
Andreas Thierfelder, spokesperson for Monsanto Agrar Germany, said Greenpeace had already been unsuccessful in several attempts to question studies done on the effects of MON863 in feed. "But the allegations were refuted every time by competent authorities," Thierfelder said. He said the European Food Safety Authority and the German Office of Consumer Protection and Food Safety had evaluated Monsanto's experiments with GM feed. Monsanto Germany's spokesman said the authorities had found that "MON863 to be as unquestionable for health and the environment as conventional maize."
New study reveals signs of toxicity of GE maize approved for human consumption - Greenpeace demands immediate withdrawal of high-risk GE products
Greenpeace Intl. - Press Release, Mar 13 2007 - http://www.yubanet.com/artman/publish/article_52782.shtml
Laboratory rats, fed with a genetically engineered (GE) maize produced by Monsanto, have shown signs of toxicity in kidney and liver, according to a new study.(1) This is the first time that a GE product which has been cleared for use as food for humans and animals has shown signs of toxic effects on internal organs. The study, published today in the journal "Archives of Environmental Contamination and Toxicology", analysed results of safety tests submitted by Monsanto to the European Commission when the company was seeking authorisation to market its GE Maize variety MON863 in the EU. (2). The data shows that MON863 has significant health risks associated with it; nonetheless, the European Commission granted licences to market the maize for consumption by both humans and animals. (3)
The incriminating evidence was obtained by Greenpeace following a court case (4), and passed on for evaluation by a team of experts headed by Professor Gilles Eric Seralini, a governmental expert in genetic engineering technology from the University of Caen. (5) In a joint press conference with Greenpeace at Berlin, Professor SEralini said, "Monsanto's analyses do not stand up to rigorous scrutiny " to begin with, their statistical protocols are highly questionable. Worse, the company failed to run a sufficient analysis of the differences in animal weight. Crucial data from urine tests were concealed in the company's own publications." Greenpeace is demanding the complete and immediate withdrawal of Monsanto's MON 863 maize from the global market and is calling upon governments to undertake an urgent reassessment of all other authorised GE products and a strict review of current testing methods. "This is the final nail in the coffin for the credibility of the current authorisation system for GE products. Once it's known that a system designed to protect human and animal health has approved a high-risk product despite clear evidence of its dangers, we need to start "strip-searching" all GE products on the market, and immediately abort this flawed approval procedure," said Christophe Then, Genetic Engineer campaigner, Greenpeace International.
The data in question has been the subject of fierce debate since 2003, when significant changes were identified in the blood of tested animals fed on MON863. MON863 was approved by the European Commission, in spite of opposition by a majority of EU member states, who raised concerns over the safety of the maize. Professor Seralini's analysis now scientifically confirms these concerns. As the study states, "with the present data, it cannot be concluded that GM corn MON863 is a safe product." And yet, MON863 has been authorised for markets in Australia, Canada, China, Japan, Mexico, the Phillipines, and USA, besides the EU. "This is an international emergency alert, requiring a global response," concluded Then, "Only a complete withdrawal from all markets will curtail the possible damage."
Notes:
1. The article is due to be published online (http://www.springerlink.com/content/"k=1432-0703) by the American journal Archives of Environmental Contamination and Toxicology; it will be printed in May. A copy can be faxed on request. A Greenpeace briefing on the study is available at: http://www.greenpeace.org/international/press/reports/gp_briefing_seralini_study
2. The tested GE maize named MON 863 produces a new insecticide called "modified Cry3Bb1" able to kill a pest insect in the soil (Diabrotica virgifera). This GE maize also contains a gene coding for antibiotic resistance.
3. The European Commission granted a license for MON 863 to be used in feed in August 2005, and subsequently approved it for human consumption in January 2006.
4. For details, please refer to the Greenpeace paper: "The MON863 case -a chronicle of systematic deception" http://www.greenpeace.org/international/press/reports/mon863_chronicle_of_deception
5. The analysis team was headed by Professor Seralini from the University of Caen and included experts from the French independent scientific organisation CRIIGEN.
Strong Suspicions of Toxicity in One GMO Corn - By Stephane Foucart - Le Monde, March 14 2007
http://freddevan.com/wordpress/2007/03/13/strong-suspicions-of-toxicity-in-one-gmo-corn/
[for the French original http://au2007alenconbis.canalblog.com/archives/2007/03/13/4297922.html]
Allowed to go on the market in France and Europe, MON 863, a transgenic corn invented by Monsanto, has been at the center of a controversy over its innocuousness for over two years (April 23rd, 2004, Le Monde). These debates could resume after the March 13th publication in "Archives of Environmental Contamination and Toxicology" of a study suggesting this genetically modified organism (GMO) is toxic to the liver and kidneys.
According to this work, consumption of MON 863 corn disturbs numerous biological parameters in rats to a greater or lesser extent: weight of the kidneys, weight of the liver, the level of reticulocytes (new red blood cells), the level of triglycerides, etc. Urinary chemistry is also changed, with reductions in excreted sodium and phosphorus going as high as 35 percent. The effects vary with the sex of the animals. "Female rats exhibit an increase in blood fat and sugar levels, and an increase in body weight - all associated with greater hepatic sensitivity," says Mr. Seralini, principal author of this study and, moreover, president of the Research Committee for Independent Research and Information on Genetic Engineering (Criigen). "Among males, the impact is opposite, with a drop in body and kidney weights."
The authors of this work used data drawn from an experiment sponsored by Monsanto, which bore on the study of 400 rats for 90 days. The statistical treatment applied to these data by the experts of the agrochemical firm was published in August 2005, by "Food and Chemical Toxicology." That work brought to light significant variations in biological parameters between animals fed MON 863 and those fed with its isogene - the same plant variety without the genetic modification. Monsanto researchers, for their part, had concluded that those disparities were within the frame of the natural variability of the measured parameters. The effects produced by the GMO were therefore not considered pathological. As for the "natural variability," it had been established by measuring the same series of data on rats fed with other varieties of non-GMO corn, with different nutritional values from MON 863 and its isogene.
The raw experimental data - over a thousand pages - were kept confidential by the agrochemical firm until Greenpeace obtained an order for its publication in spring 2005 from the Appeals Court of Munster (Germany).
Criigen was thus able to examine the data in detail and to apply a new statistical treatment to them. According to Mr. Seralini, that, notably, consisted of extracting from the raw data the most significant effects specifically imputable to GMO absorption.
"Of the 58 parameters measured by Monsanto," the researcher details, "all those that were altered concern kidney or liver functioning." He continued, "furthermore, Monsanto had deemed that, because the males and the females responded differently, there was no reason for worry." He added, "Yet, the liver, for example, is an organ that reacts differently as a function of sex." In the same way, the fact that the measured biological response was not always in exact correlation with the dose of GMO received was interpreted by the company's experts as proof that the transgenic corn being tested was not the cause. Mr. Seralini contests that principle: "When the disturbances are hormonal, for example, the impact may not be proportional to the dose."
Toxicologist Gerard Pascal, a member, like Mr. Seralini, of the Committee on Bio-molecular Engineering, deems certain that Criigen's conclusions are erroneous. "I reject the analysis of the animals' weight curves, conducted without taking their feeding into account," says Mr. Pascal. "But I agree that the biological responses may vary between males and females and with the principle that the effects of a GMO corn must be compared with its isogene only and not take into account effects produced by other corn varieties."
According to Mr. Pascal, the lack of direct correlation between the GMO doses received and the impacts observed on the hepatic parameters disqualifies the conclusions about liver toxicity. Significant differences with respect to "kidney weight" and "urinary sodium, phosphorus, and potassium" suggest a renal impact. "However," Mr. Pascal recalls, "at my request, the CGB pressed for investigations of the kidneys and had not found any definitive evidence of toxicity" (December 15th, 2004, Le Monde). "The variations in the levels of reticulocytes and eosinophiles (white blood cells) remain," adds M. Pascal. "I don't know how to interpret that, but those are parameters that move around a lot in experiments." As far as Mr. Pascal is concerned, the information developed by Criigen is not of a nature to call into question the favorable opinions delivered with respect to MON 863. "All that is nothing but a personal interpretation," adds the toxicologist.
Criigen's work has been financed by Carrefour and Greenpeace, but, as Mr. Seralini explains, "Unfortunately, today there is no public budget for conducting this type of research." A situation all the more harmful, according to Mr. Seralini, in that, "the whole toxicological study ought to be redone, controlling for hormonal dosages" and, above all, the tests should be continued well beyond 90 days and on species other than the rat to reach a definitive conclusion.
(Translation: t r u t h o u t French language correspondent Leslie Thatcher)
New setback for biotech crops in Europe - By Andrew Bounds in Bonn - Financial Times - March 13 2007
The battle over biotech crops erupted again yesterday after members of the European parliament blocked a resolution calling for greater use of the technology. MEPs voted to delay the draft motion to allow more time for the agriculture committee to scrutinise it.
The Socialist group, the second-biggest in parliament, said: "It needs more debate to be better balanced and flexible." The cross-party vote deals a blow to efforts by the European Commission to boost biotechnology at a ministerial meeting in June that will set new targets for its use.
The resolution by Kyösti Virrankovski, a Finnish MEP, called for the benefits of genetic modification to be recognised and for an end to discrimination between GM and conventional crops. Of 90m hectares planted worldwide in 2005, 65,000ha were in the EU.
Industry advocates say the delay in Europe is costing jobs and investment as the US and Asia plant crops. CBAG, an advisory group to the Commission of scientists and industry figures, said the Commission "should calculate the negative effect on employment and competitiveness of delay". It also called for compensation for patent holders who could not get national governments to allow planting.
A recent report by an outside consultant for the US Grains Council showed that farmers gained $5bn extra in 2005 by cutting down on pesticides and ploughing. The crops are resistant to weeds so ploughing is reduced, saving on fuel and the release of carbon dioxide into the atmosphere.
Simon Barber of EuropaBio, the industry lobby group, said: "If they didn't work, why would 10m farmers plant them?" However, Friends of the Earth said early benefits of new crops often evaporated within a few years as new diseases or pests adapted. The pressure group issued a report questioning the benefits of GM crops. Hundreds had been approved in the US but only 70 different ones had been planted.
"If we want to develop a competitive and dynamic economy in Europe, then it would be wise to quietly shelve the idea of genetically modified foods and put our political support and tax-payers' money behind green farming methods," said FoE.
All eyes are on the European Commission's joint research council of scientists' study, due next month. Early drafts say there is not enough data to assess but that GM crops accounted for just 0.08 per cent of gross valued added in the agricultural food industry and 0.02 per cent of jobs in the EU. However, that is because few have been planted amid consumer resistance. Polls show seven in 10 Europeans oppose GM crops, causing governments to withhold approval and retailers to avoid stocking them.
Copyright http://www.ft.com/servicestools/help/copyright The Financial Times Limited 2007
GM SPUDS HAVE HAD THEIR CHIPS AS IRISH TRIALS STOPPED - By Aideen Sheehan - Irish Independent, 12 March 2007
CHEMICAL giant BASF has abandoned its plans to grow genetically modified potatoes in Ireland. It is now opting to grow them in Britain where there are fewer restrictions. A company spokesperson confirmed that the company would not be going ahead with field trials in Co Meath which received approval from the Environmental Protection Agency (EPA) last year.
BASF delayed starting the trial last year citing the onerous monitoring requirements imposed by the EPA. The firm said at the time that it would assess whether it could find a way to proceed in 2007. However, a spokesperson has confirmed to the Irish Independent that the company has decided to abandon the Irish experiment and has opted to trial the GM potatoes in Britain instead, provoking the ire of environmentalists there. The potatoes are genetically altered to improve resistance to blight, the most serious potato pest, with opponents claiming they could contaminate conventional crops.
"We don't need GM potatoes and there is no consumer demand for them. The Government should promote safe and sustainable agriculture, not this half-baked GM potato plan," said Friends of the Earth campaigner Clare Oxborrow.
Although the GM experiment is slow to take off in Ireland, the GM-Free Ireland network claimed last week that Agriculture Minister Mary Coughlan is determined to legalise the release of GM crops after the general election. Regulations on the co-existence of GM and conventional crops are expected to be finalised, but opponents want controls tight enough to make it almost impossible to grow GM food.
A number of county councils around the country have declared themselves GM-free zones, but this has no legal power as the EPA is the body charged with approving the cultivation of GM organisms. However, official figures show that hundreds of thousands of tonnes of genetically modified (GM) animal feed are now being imported each year. Up to 95pc of all the maize and soya brought into the country for use as animal feed is genetically modified, which is legal as long as it is correctly labelled. Some 464,000 tonnes of GM maize, 204,000 tonnes of GM soya and 4,300 tonnes of GM rape-seed were imported last year.
Dutch Council of State ordered destruction of BASF GE potato field trial - Linda Coenen, ASEED (Netherlands), 9 March 2007
http://www.gene.ch/genet/2007/Mar/msg00031.html
Last Wednesday, March 7th, The Council of State in The Netherlands judged in a appeal by Greenpeace that the field trials of BASF had been illegally permitted by the Ministry of Housing, Spacial Planning, andnEnvironment (VROM) and destroyed the permits immediately. The court decision was based on the grounds that 1) these potatoes had been insufficiently tested in a controlled environment (like a greenhouse or laboratory) to be release in the open, and 2) the Ministry had not been able to do a proper environmental effect assessment (as required) since BASF had failed to provide information specific enough for this purpose on the location of the trial sites. It concerns three BASF GM potato varieties, two with changed starch content similar to the Amflora-potato and one with hightened late blight resistence. All three are also herbicide-resistent.
Court decision (Dutch): http://www.raadvanstate.nl/verdicts/verdict_details.asp?verdict_id=16397
Greenpeace press release (Dutch): http://www.greenpeace.nl/news/rechter-trekt-vergunning-proef
Trial descriptions:
http://gmoinfo.jrc.it/gmp_report.aspx?CurNot=B/NL/05/03
http://gmoinfo.jrc.it/gmp_report.aspx?CurNot=B/NL/05/04
http://gmoinfo.jrc.it/gmp_report.aspx?CurNot=B/NL/05/05
GM starch potato: still no cultivation in 2007 - GMO Compass, Germany, 5 March 2007
http://www.gmo-compass.org/eng/news/messages/200703.docu.html#97
The Amflora potato, developed by BASF Plant Science with an altered starch composition, apparently may not yet be cultivated this year in the EU. As reported by the magazine Agrar Europe, the European Commission has requested an opinion from the European Medicines Agency, EMEA, as prerequisite to an approval decision.
The subject of interest is the marker gene used in the potato, making it resistant against the antibiotic kanamycin. GM plants are only approved in the EU, if the containing resistance gene has no harmful effects on health and environment. According to a current study by the World Health Organisation, WHO, the relevant antibiotic kanamycin may have a greater importance in veterinary medicine than has been assumed to date. However, the European Food Safety Authority has already identified no safety concerns which may have an adverse effect upon approval.
The Amflora potato contains only starch with the amylopectin component, and delivers renewable raw material to the starch industry. Its cultivation was planned already for 2007. Three cultivation areas have been registered provisionally in the site register of the Federal Bureau for Consumer Protection, BVL.
GMO-Compass: Amflora approval - http://www.gmo-compass.org/eng/gmo/db/17.docu.html
GMO-Compass: Why Antibiotic Resistance Genes in Transgenic Plants? - http://www.gmo-compass.org/eng/safety/human_health/45.docu.html
GMO-Compass: Alternatives to Antibiotic Resistance Marker Genes - http://www.gmo-compass.org/eng/safety/human_health/129.docu.html
GM CROPS FAIL EU LISBON AGENDA GOALS - Friends of the Earth Europe - PRESS RELEASE - March 12th 2007
New research says green farming more competitive
Brussels, March 12th 2007 - Environmentally-friendly farming will create more jobs and make the EU more competitive than if it grows genetically modified (GM) crops, shows new research published today by Friends of the Earth Europe. The research coincides with the expected withdrawal later today of a European Parliament Resolution that promotes GM crops. MEPs are requesting that the text be rewritten because it attacks the precautionary principle and ignores research showing that GM food and farming has not lived up to expectations [1].
Helen Holder, European GMO campaigner at Friends of the Earth Europe, said: "The genetic modification approach to farming is failing despite the hype, public funding and political will. Greener farming stimulates the economy, benefits the environment and the public loves it."
Today's report launched by Friends of the Earth Europe [2] highlights:
- A lack of official data on how badly the agri-biotechnology sector is performing despite high levels of public funding and high political priority in key EU areas such as Enterprise and Research. After 25 years of public EU funding for research, only two GM traits have ever been commercialised on any scale.
- Evidence of increased social cohesion, rapid growth and job creation in the environmentally-friendly farming and food sector, for example in organics; compared with virtually no jobs, de-investment and lack of profits by companies developing GM crops and foods.
- The sidelining of greener farming due to political support for GM crops and foods, despite its better economic performance and environmental credentials such as using less energy, less water and fewer pesticides.
- The threat of economic damage to green farming and food production from contamination by GM crops
The report comes as the EU is preparing new targets for biotechnology as part of the mid-term review of its Biotech Strategy, which will be adopted by the EU Competitiveness Council in June [3]. Friends of the Earth Europe insists that it is economically unjustified to further promote GM crops and foods and that this must be recognized in the revised EU Biotech Strategy.
"If we want to develop a competitive and dynamic economy in Europe then it would be wise to quietly shelve the idea of genetically modified foods and put our political support and tax-payers money behind green farming methods, which can deliver. Environmentally-friendly agriculture is not only being sidelined in the doomed quest for a biotechnology solution, it is even under threat due to the risks of contamination from GM crops," Ms Holder added
For more information, please contact:
Helen Holder, GM Campaigner at Friends of the Earth Europe: Tel : +32 2 542 0182, Mobile +32 474 857 638, Email: helen.holder@foeeurope.org
Rosemary Hall, Communications Officer at Friends of the Earth Europe: Tel: +32 25 42 61 05, Mobile: +32 485 930515, Email: rosemary.hall@foeeurope.org
NOTES:
[1] Resolution A6-0032/2007 "Biotechnology: Prospects and challenges for agriculture in Europe", rapporteur: MEP Kyösti Virrankoski
[2] "The EU's Biotech Strategy: Mid-term review or mid-life crisis? A scoping study on how European agricultural biotechnology will fail the Lisbon objectives and on the socio-economic benefits of ecologically compatible farming" Friends of the Earth Europe, March 2007
Executive summary: http://www.foeeurope.org/publications/2007/FoEE_biotech_MTR_midlifecrisis_March07_execsum.pdf
Full report: http://www.foeeurope.org/publications/2007/FoEE_biotech_MTR_midlifecrisis_March07.pdf
[3] The EU Biotech Strategy was adopted in 2002: http://ec.europa.eu/biotechnology/pdf/com2002-27_en.pdf. The European Commission will issue its recommendations for the Mid Term Review in April 2007 and conclusions will be adopted by the June 2007 Competitiveness Council.
Rosemary Hall, Communications Officer, Friends of the Earth Europe, Rue Blanche 15, B-1050 Bruxelles, Belgium
Tel.: +32 2 542 6105 Mobile: +32 485 930515 Fax: +32 2 537 5596 rosemary.hall@foeeurope.org - http://www.foeeurope.org
European ministers uphold Hungary's right to ban GMO crop - Agence France Presse - February 20, 2007 - posted by blilliston@iatp.org
European environment ministers on Tuesday upheld Hungary's right to ban a genetically modified product (GMOs), dealing a policy defeat to the EU's executive arm which wanted the measure to be lifted. A "qualified majority" of the 27 EU member states rejected the European Commission demand that a "safeguard clause" which Budapest invoked in 2005 to keep Monsanto GMO maize out of the country be lifted. The maize has been authorised for use in the EU since 1998. The ministers made exactly the same decision on the same maize on Austria's behalf in December.
Environmental group Greenpeace hailed the environment ministers' "bold decision". "We look forward to the day when the European Commission also puts defence of the public interest before the interests of US agribusiness and its lobbyists in Brussels and at the WTO," said Marco Contiero, policy adviser on GMOs at Greenpeace European Unit.
Under EU legislation, a member state has the right to apply a temporary safeguard clause against GMO products if it can provide scientific evidence placing their safety in doubt. But the European Food Safety Authority has judged that the evidence in the Austrian and Hungarian cases is "scientifically unfounded" and said there was no reason to fear any related health problems for people, animals or the environment. Strengthened by this advice, the Commission called on the member states to force Hungary, one of the EU's biggest grain producers, to lift the clause, but in vain. Brussels must now decide whether to drop the issue or to come up with a new proposal in a bid to overcome member states' misgivings. The EU risks a conflict with the World Trade Organisation which a year ago charged that the safeguard clauses were not justified scientifically.
EU ENVIRONMENT MINISTERS TWICE RESIST GMOs
Friends of the Earth Europe - Press Statement - Tuesday 20th February 2007
Brussels - Today, Environment Ministers from EU Member States voted to allow Hungary to uphold its national ban of Monsanto’s genetically modified maize [1].
Helen Holder, GMO campaigner at Friends of the Earth Europe said, “EU countries have defended Hungary’s right to protect its environment and its citizens’ health by banning a genetically modified crop. Bans such as Hungary’s are allowed under EU law and according to the World Trade Organisation rules and EU countries were quite right to refuse to
be bullied by the European Commission into annulling the ban.”
Environment Ministers also failed to give the green light for the marketing of a genetically modified flower. Since the Ministers failed to reach a clear agreement amongst themselves, the final decision, under EU rules, will now revert back to the European Commission.
For more information, please contact:
Helen Holder, GM Campainger at Friends of the Earth Europe : Tel : +32 2 542 0182 , Mobile +32 474 857 638 , Email : helen.holder@foeeurope.org
Rosemary Hall, Communications Officer at Friends of the Earth Europe: Tel:+32 25 42 61 05 , Mobile: +32 485 930515 , Email: rosemary.hall@foeeurope.org
NOTES
[1] Genetically modified maize, MON810, produced by Monsanto. Prohibited by Hungary under the Safeguard Clause of Directive 2001/18